No. 23 Analysis of Fatty Acid in α-Cyclodextrin
Summary
α-Cyclodextrin (α-CD) is now industrially produced and widely used in various application fields. The turbidity of α-CD aqueous solution has been sometimes observed in spite of much higher water solubility of α-CD (14% at 25℃) than that of β-CD (2% at 25℃). The cause of the turbidity has long been uncertain. Here we report on our finding that small amounts of fatty acids coming from the raw material, corn starch are a potential source of the turbidity.

Study purpose
- CAVAMAX® W6 FOOD (Wacker Chemie) was used as an industrially produced α-CD. The oil fraction in α-CD was extracted by an organic solvent, and the fatty acid component was determined by an official method of food analysis using GC-MS.
- The turbidity of saturated α-CD solution was evaluated by visual observation and visible adsorption at 660 nm. The effects of the fatty acid component in α-CD on the turbidity of the solution were investigated.
Tested α-CDs
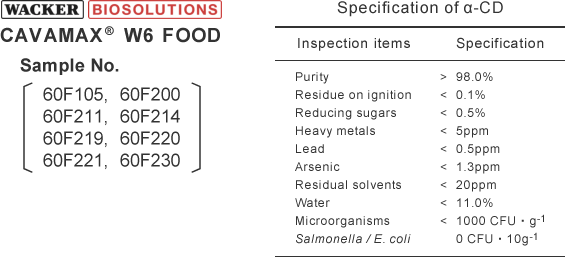
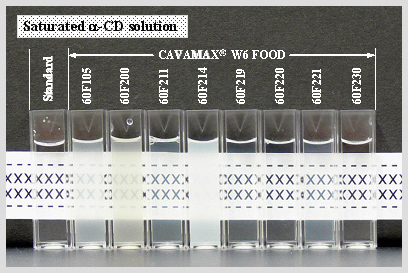
Evaluation method of turbidity of saturated α-CD solutions by visual observation
Evaluation method of turbidity of saturated α-CD solution
Fatty acid component of oil fraction in α-CD
Fatty acid component of oil fraction extracted from α-CD
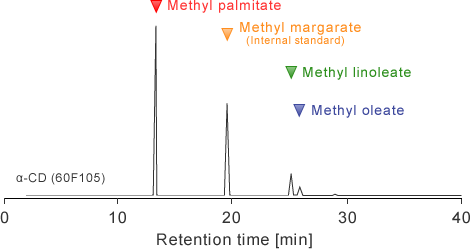
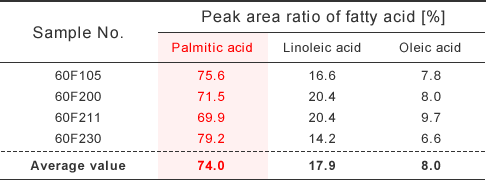
A small amount of palmitinic acid, linoleic acid, and the oleic acid was observed in the industrially produced α-CD, and the fatty acid component was 74.0%, 17.9% and 8.0% respectively. The big differences were observed in fatty acid component between α-CD and a corn starch(10%, 59%, 26%)of the raw material.
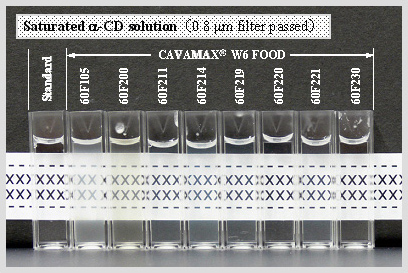
Visual evaluation of saturated α-CD solutions
Turbidity of saturated α-CD solutions evaluated by visual observation
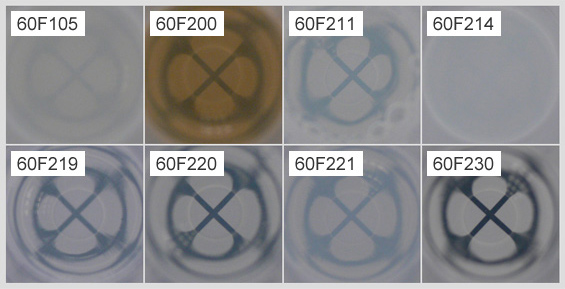
Saturated α-CD solution (0.8 mm filter passed)
The differences of the turbidity and the color tone were observed depending on the production lots.
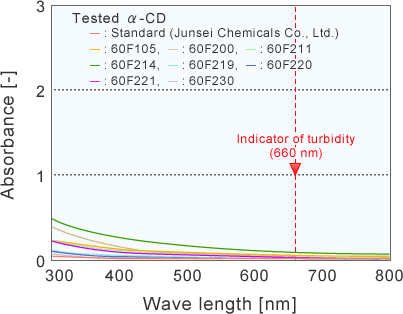
Turbidity of saturated α-CD solutions evaluated by UV-Vis spectrum
UV spectrum of saturated α-CD solutions
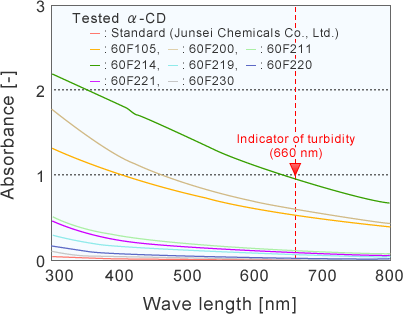
Cell size : 9 x 9 x 44 mm
The visible adsorption of an industrially produced α-CD at 660 nm was 1.9~107.8 times higher than that of an assay reagent of α-CD.
Turbidity of saturated α-CD solutions after filtration evaluated by UV-Vis spectrum
UV spectrum of saturated α-CD solutions after passing 0.8 mm filter
Cell size : 9 x 9 x 44 mm
The turbidity of saturated α-CD solution was markedly decreased by 0.8 mm filtration.
Turbidity change of saturated α-CD solutionsbefore / after filtration
Change of absorbance at 660 nm before / after passing 0.8 mm filter
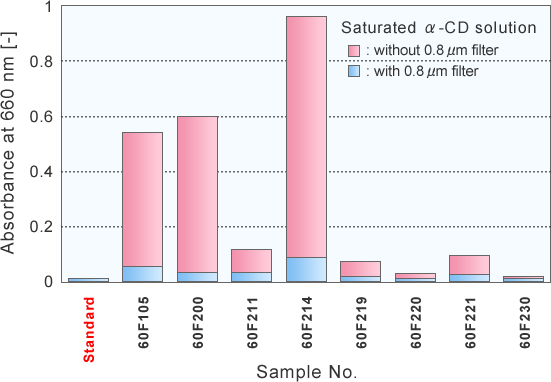
The turbidity of saturated α-CD solution might be originated by suspended microparticle in the solution.
Relationship between oil fraction content in α-CD and turbidity of saturated α-CD solution
Oil fraction content in α-CD and turbidity of saturated α-CD solution
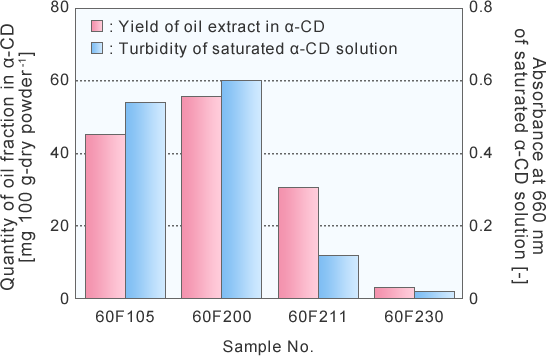
A significant correlation was observed between the quantity of oil fraction in α-CD and the turbidity of saturated α-CD solution.
Comparison of water solubility of α-CD of different production lots
Water solubility of saturated α-CD solution
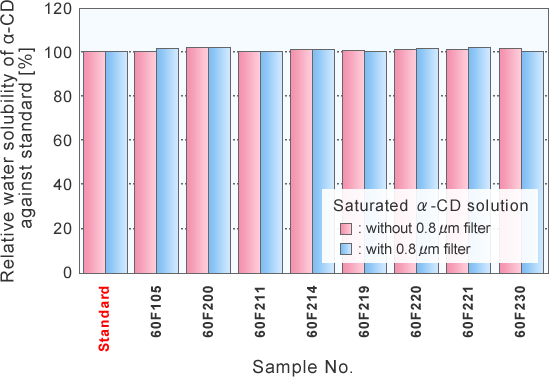
Since the amount of the microparticle in the saturated α-CD solution was very little, the water solubility of the industrially produced α-CD was hardly changes compared with an assay reagent of α-CD
Conclusion
- A small amount of palmitinic acid, linoleic acid, and the oleic acid was observed in the industrially produced α-CD, and the fatty acid component was 74.0%, 17.9% and 8.0% respectively. The big differences were observed in fatty acid component between α-CD and a corn starch(10%, 59%, 26%)of the raw material.
- The visible adsorption of an industrially produced α-CD at 660 nm was 1.9〜107.8 times higher than that of an assay reagent of α-CD.
Since the turbidity was decreased after passing 0.8 mm filtration, it might be originated by microparticle. -
A significant correlation was observed between the quantity of oil fraction in α-CD and the turbidity of saturated α-CD solution.
→ It is reported that the binding constant between α-CD and a fatty acid increases by an increase of hydrophobic strength of fatty acid1). Therefore, it would be very difficult to remove palmitic acid in the industrial manufacturing of α-CD, and the turbidity of saturated α-CD was originated by the complex between α-CD and palmitic acid.
1) T. Yamamoto, N. Taira, Y. Akihara and Y. Matsui, 20th Cyclodextrin Symposium(2002, Chiba, Japan) pp. 124-125
