No. 34 Effect of Complexation of R(+)-α lipoic acid with Cyclodextrin on the Aqueous Solubility and Radical Scavenging Activity.
Introduction
Alpha-lipoic acid as Antioxidant
In 1959, Rosenberg, et al reported that the administration of alpha-lipoic acid to the guinea-pig which had ascorbic acid deficiency improved its symptom of scurvy and they also reported that the dosage of alpha-lipoic acid to the rat which was given the diet without Vitamin E prevented Vitamin E Deficiency1).
Later, it was reported that alpha-lipoic acid had effect on the diabete neuropathy2) or some other neuropathy3,4) and the association between free radicals and these neuropathy focused on the antioxidant activity of alpha-lipoic acid.
1) Rosenberg, H. R., Culik, R. : 「Effect of α-lipoic acid on vitamin C and vitamin E deficiencies」 Arch. Biochem. Biophys., 80, 86-93 (1959)
2) Jorg, J. Metz, F., Scharafinsky, H. : 「Drug treatment of diabetic polyneuropathy with alpha-lipoic acid or vitamin B preparations. A clinical and neurophysiologic study」 Nervenartz., 59, 36-44 (1988)
3) Altenkirch, H., Stoltenburg-Didnger, H., Wagner, M., Herrman, J., Walter, G. : 「Effects of lipoic acid in hexaxarbon-induced neuropathy」 Neurotoxicol. Treat., 12, 619-622 (1990)
4) Prehn, J. H. M., Karkoutly, C., Nuglisch, J., Peruche, B., Krieglstein, J. : 「Dihydrolipoate reduces neuronal injury after cerebral ischemia」 J. Cer. Blood Flow Metabol., 12, 78-87 (1992)
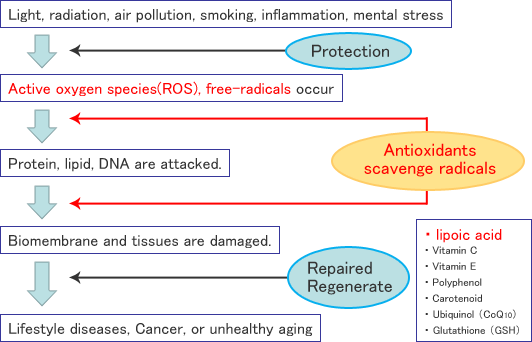
Defense against oxidative stress
What is alpha-lipoic acid
Alpha-Lipoic acid (ALA) is one of the 5 important coenzymes, which exists in the mitochondrion linked with protein and takes part in the metabolic pathway. ALA has chiral atom on C6* (Fig.1) and exists as two enantiomers; R(+)-alpha-lipoic acid (RALA) and S(-)-alpha-lipoic acid (SALA). RALA is the only naturally occurring lipoic acid and works as coenzyme.
RALA and its reduced form, R(+)-Dihydrolipoic acid(R-DHLA) are coupling in vivo and make expression of their antioxidant activity.
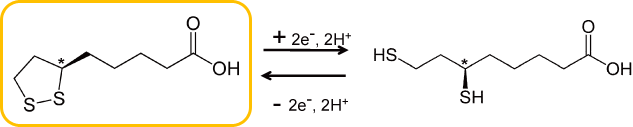
Thiol/disulfide exchange by lipoic acid is the basis for its modulation of the cell's energy and redox status.
Antioxidant activity of lipoic acid
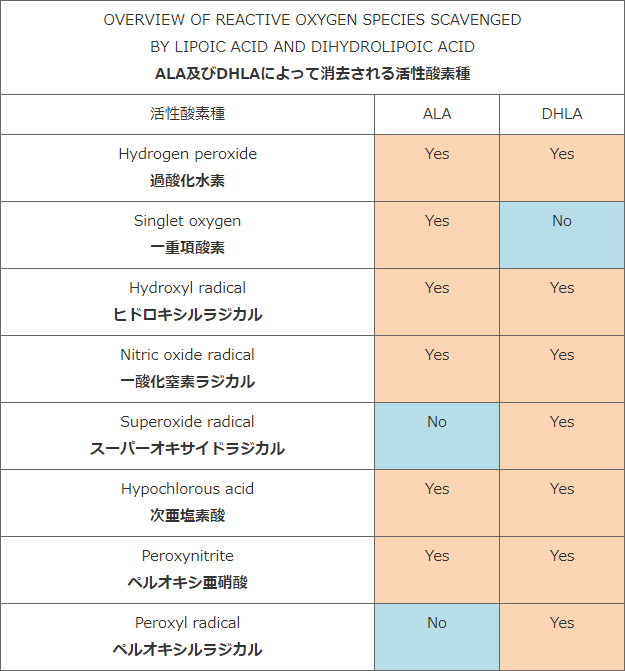
5) Packer L., Kraemer L., Rimbach G.:「Molecular aspects of lipoic acid in the prevention of diabetes complications」 Nutrition , 17(10), 888-895 (2001)
Antioxidant network
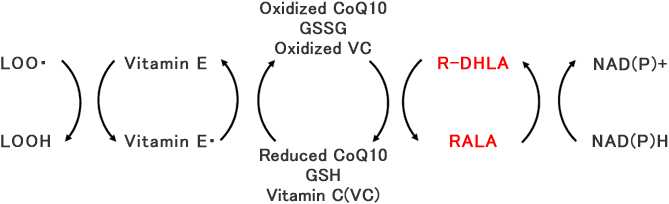
小西徹也、小久保晋、松郷誠一:「機能性生理活性物質リポ酸の化学と新素材としての可能性」日本食品新素材研究会誌、8(2)51-64 (2005)
Oxidation reduction : ORP
Oxidized glutathione GSSG + 2e- → 2GSH: −0.24V
Ubiquinone (CoQ10) (Eo') :+0.10V
Lipoic acid ALA +2e- → DHLA : −0.32V
RALA itself has antioxidant activity and, furthermore, plays a important role to restore other antioxidants by coupling with DHLA. In this way, it keeps balance between oxidative stress and scavenging activity.
Stabilized R(+)-α lipoic acid
RALA is the only naturally occurring lipoic acid, but it is very unstable to form polymeric compound by external stimuli such as heat, photolysis and so on.
For this reason, it has been difficult to use RALA as a neutraceutical fields. Now in the market, racemic mixture, which contains R(+) form and S(-) form at the ratio 1:1, is used for supplement or health food.
Melting point
- Racemic mixture: 60-62℃
- R(+) form: 46-49℃
→ Therefore, stabilization of R(+)-α lipoic acid is needed.
We succeeded in developing stabilized RALA by using Cyclodextrin against heat and acidic condition.
Purpose
RALA scavenges ROS and free radicals, which cause oxidative stress.
The radical scavenging activity of ALA is well known, but the physico-chemical properties of RALA-CD complex has been scarcely reported so far.
↓
In this study, aiming to evaluate physico-chemical properties of stabilized RALA, which is inclusion complex of RALA with Cyclodextrin(CD), its solubility into water and DPPH radical scavenging activity were investigated.
Experiment - Solubility into water
Procedure
- Each amount of RALA-CD; 25mg, 50mg, 75mg, 100mg, is added to 5ml of non-ion water.
- Mixed for 30min in the bath at temp.=70℃ and left until it is cooled to RT.
- Filter the sample suspension using 0.2μm membrane.
- Assay %RALA in the filtered sample solution by HPLC.
Result
There was the range in which RALA concentration increased linearly (Fig.3).

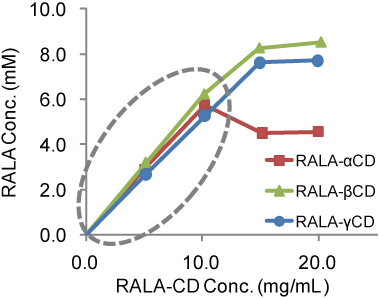
Experiment - Binding constant
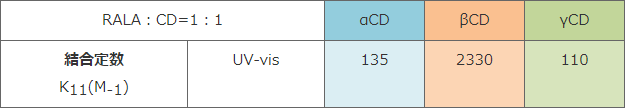
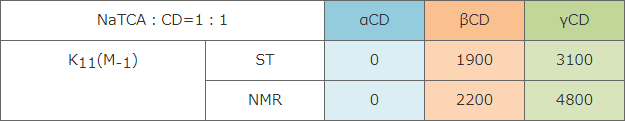
7) P.R. Cabrer et al. : 「Complexation of Bile Salts with α-, β- and γ-Cyclodextrins. Effects of the Cavity Size in the Complexation of Steroids.」
25oC and pH7 in different concentrations of CDs.
Experiment - radical scavenging activity
Procedure
Sample solution, in which CD or RALA-CD is solved into MilliQ, is mixed with DPPH ethanol solution and soon after the reaction occurred, DPPH radical's spectrum is measured by ESR.
Result
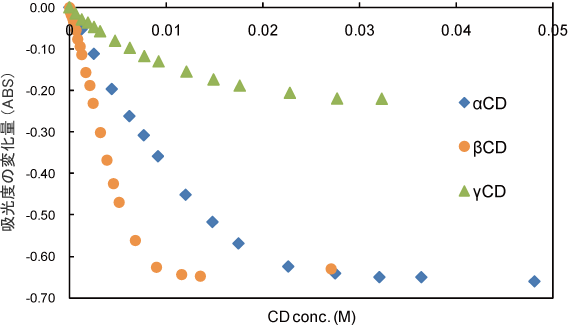
In this experimental system, CD showed no DPPH radical scavenging activity (Fig. 5).
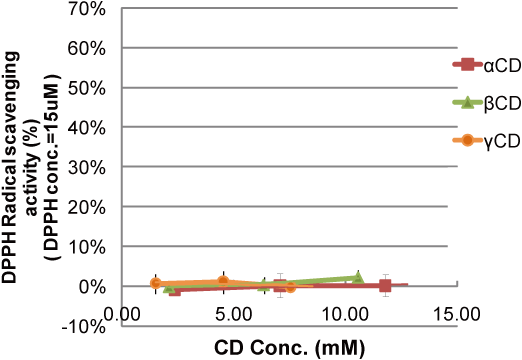
Experiment - radical scavenging activity
Result
RALA-CD showed higher DPPH radical scavenging activity than R(+)-α lipoic acid sodium salt (NaRALA)(Fig. 6).
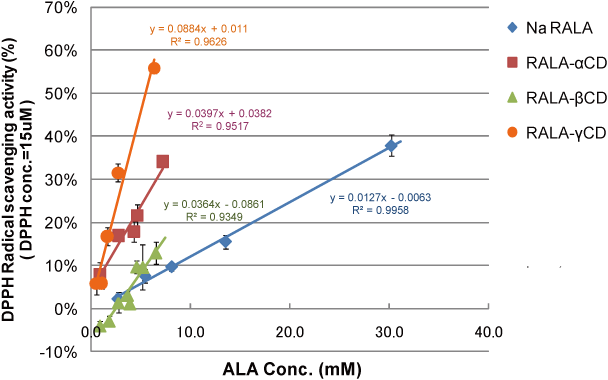

Summary
DPPH radical scavenging activity of R(+)-α lipoic acid-CD complex in this experimental system,
- RALA-CD showed higher DPPH radical scavenging activity comparing to NaRALA . And IC30 order was RALA-γ-CD > RALA-α-CD > RALA-β-CD > NaRALA
→ The smaller the binding constant of R(+)-lipoate anion with CD is, the higher the DPPH radical scavenging activity is.
Compared to binding constant of NaTCA with CD, the binding constant of lipoate anion with α-CD or γ-CD was smaller. This result indicates that RALA- α-CD or RALA- γ-CD can release RALA molecule in the intestines. - CD itself showed no DPPH radical scavenging activity.
→ This result indicates that the enhancement of RALA-CD complex radical scavenging activity compared to NaRALA is owing to CD complexation.
